Abstract
Introduction Chimeric antigen receptor T (CAR T) cells targeting CD19 and BCMA are active in the treatment of B cell lymphoid malignancies. CAR T cell therapy can be complicated by cytokine release syndrome (CRS), a syndrome characterized by systemic inflammatory response, in which interleukin (IL) -6 plays a central role. Tocilizumab is a monoclonal antibody targeting the IL-6 receptor and is recommended for treatment of CRS. A national shortage of tocilizumab occurred in 2021. In order to conserve supply and continue providing CAR T cell therapy, our center replaced tocilizumab with siltuximab in our CRS management algorithm. Siltuximab is a monoclonal antibody that binds to IL-6 itself. We report the outcomes of patients treated with this "siltuximab-first” (SF) approach in comparison with patients treated with a "tocilizumab-first” (TF) based approach.
Methods We conducted a retrospective review of patients treated with CAR T cells for lymphoma and multiple myeloma. Baseline characteristics and outcomes were collected from the transplant and cellular therapy database and medical records. CRS was graded according to the Lee scale and immune effector cell-associated neurotoxicity syndrome (ICANS) was graded according to the CAR T cell toxicity management grading system. At our center, anti-IL-6 therapy is utilized for grade 2 or greater CRS, or persistent grade 1 per provider discretion. The length of stay for CAR T hospitalization was measured from the day of CAR T cell infusion. Comparisons between groups were done using Fisher's exact test for nominal variables and Kruskal-Wallis rank sum test for continuous variables. All analyses were done using R and its packages.
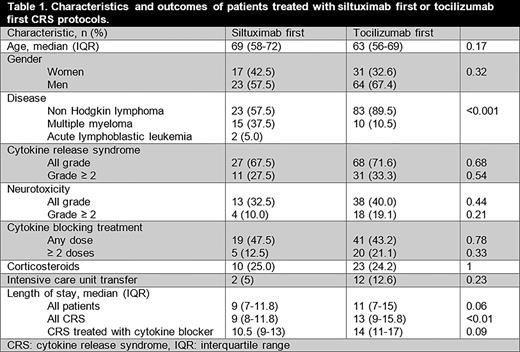
Results We identified 135 patients treated with CAR T cell therapy. Baseline characteristics are listed in Table 1. The majority of patients received treatment for relapsed or refractory B cell non Hodgkin lymphoma (NHL).
CRS was observed in 95 (70.4%) patients, 44 (32.5%) had grade ≥ 2 CRS. Patients treated with the SF and TF protocols had comparable rates of CRS (SF = 67.5% vs. TF = 71.6%, p = 0.68) as well as grade ≥ 2 CRS (SF = 27.5% vs. TF = 33.3%, p = 0.54). Fifty one (37.8%) patients developed neurotoxicity, 23 (17%) grade ≥ 2, without differences between groups.
Anti-IL-6 therapy was prescribed to 60 (44.4%) patients, without significant differences between groups (SF = 47.5% vs. TF = 43.2%, p = 0.71). The median number of doses of cytokine blocking agents was 1 (IQR 1 - 2), without a significant difference between groups. A larger proportion of patients with MM were treated using the SF CRS protocol (37.5% vs. 10.5%, p <0.001). Fourteen out of 19 patients (73.7%) treated with SF required only one dose of anti-IL-6 directed therapy, compared with 21/40 (51.2%) patients treated with TF. However, this difference did not reach statistical significance. Seven doses of tocilizumab were used in 5 patients during the period of SF protocol use, primarily due to persistent CRS after an initial dose of siltuximab or physician discretion. Thirty three patients (24.4%) received corticosteroids (inclusive of treatment of neurotoxicity), without differences in the proportions between groups (SF = 25.0% vs. TF= 24.2%, p = 1).
Transfer to an intensive care unit (ICU) setting occurred in 14 (10.4%) cases, without significant differences between groups. Median length of stay (LOS) for the entire cohort was 10 days (IQR 7-14). Patients experiencing CRS had longer median LOS (12 vs. 7 days, p <0.001). The median LOS of patients with CRS requiring IL-6 blockade was 10.5 and 14 days for SF and TF, respectively (p = 0.09).
Conclusions IL-6 blockade is an effective therapy for CRS. Adoption of a "siltuximab first” protocol in response to the national shortage of tocilizumab showed that the use of this agent is safe and feasible. We did not observe increased use of subsequent doses of anti-IL-6 therapy or steroids, and hospital resource utilization measures such as ICU transfer and LOS were comparable with tocilizumab - based protocols. The larger proportion of MM patients receiving the SF protocol can be attributed to timing of CAR T product approval and tocilizumab shortage. Differences in LOS are likely a result of population and time period differences. While prospective studies in larger numbers of patients may be needed, these results suggest siltuximab is a valid alternative for CRS management in CAR T cell patients.
Disclosures
Hamilton:Incyte: Membership on an entity's Board of Directors or advisory committees; Kadmon: Membership on an entity's Board of Directors or advisory committees; Nkarta: Membership on an entity's Board of Directors or advisory committees; Equilium: Membership on an entity's Board of Directors or advisory committees; Syndax: Membership on an entity's Board of Directors or advisory committees. Winter:Seagen, Inc: Consultancy, Membership on an entity's Board of Directors or advisory committees; Janssen: Consultancy, Membership on an entity's Board of Directors or advisory committees; OncLive: Honoraria. Jagadeesh:AstraZeneca: Research Funding; Seagen: Research Funding; Affimed: Membership on an entity's Board of Directors or advisory committees; Debio pharma: Research Funding; ATARA Biotherapeutics: Research Funding; Trillium Pharmaceuticals: Research Funding; Regeneron Pharmaceuticals, Inc.: Research Funding; MEI Pharma: Research Funding; LOXO Pharmaceuticals: Research Funding; Daiichi Sankyo: Consultancy, Membership on an entity's Board of Directors or advisory committees. Hill:Kite, a Gilead Company: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Travel Support, Research Funding; BMS: Consultancy, Honoraria, Research Funding; Novartis: Consultancy, Honoraria, Research Funding. Sauter:Kite Pharma Inc.: Consultancy; Karyopharm Therapeutics Inc.: Consultancy; Ono Pharmaceuticals: Consultancy; CSL Behring: Consultancy; Gamida Cell: Consultancy; BMS: Other: PI; Precision Biosciences: Other: PI; Genzyme/Sanofi: Other: PI. Caimi:Novartis: Membership on an entity's Board of Directors or advisory committees; Kite Pharma: Membership on an entity's Board of Directors or advisory committees; ADC Therapeutics: Membership on an entity's Board of Directors or advisory committees; Bristol Myers Squibb: Membership on an entity's Board of Directors or advisory committees; MEI Pharma: Membership on an entity's Board of Directors or advisory committees; Genmab: Membership on an entity's Board of Directors or advisory committees; Takeda: Membership on an entity's Board of Directors or advisory committees; Genentech: Membership on an entity's Board of Directors or advisory committees.
OffLabel Disclosure:
Siltuximab is FDA approved for the treatment of multicentric Castleman disease. In this presentation, we discuss its use in the management of Cytokine Release Syndrome.
Author notes
Asterisk with author names denotes non-ASH members.